The only purpose of this post is to be public and belong to all relevant categories — this enables WordPress to auto-generate lists of categories (it ignores ones that contain only private posts).
Author: admin
my results on calibration
I did this….
more
Message to Nick
What I want:
When a lab member (with the password, which is BSL2019) enters the password-protected part of the blog, I want them to land on a page that has tag and category menus that allow us to navigate our posts. But these links shouldn’t be viewable on the static pages that constitute the outward-facing web presence.
BSL internal materials
This is where we can post information for our group — things we want to be able to look up. Particularly nice data, examples of calibration measurements, methods of alignment. Also where we can have conversations.
Easy to insert additional blocks, including pictures.
Fixed Cell Measurement Protocol
- Put coverslip with adherent cells in the sample chamber and wash with PBS. This involves gently spraying the PBS on the coverslip with a pippette, and gently rocking the sample chamber back and forth. This should only require about 0.5 mL of PBS.
- Aspirate PBS and fill chamber with 1 mL of 4% formaldehyde. Let sit for 15 minutes at room temperature (in cell prep box, covered with a chem wipe).
- There are variances in both time and temperature (some incubate rather than using room temperature), so this is something that could be varied if desired.
- Aspirate formaldehyde and wash twice with PBS. Aspirate PBS.
- For measurements, fill with HBSS at 37C as is typical with live cell measurements.
Investigation of Mechanical Properties of Cornea via Raman
- We plan to use 10-20 pig (ideally human) corneas and take Raman Spectra. Then Professor Buckle’s lab can perform mechanical tests (modulus identification) on the same cornea samples to compare with Raman signal. We hope to see some correlation between the mechanical properties and Ramen signal, which may show up in collagen concentrations or in hydration.
- We also can take the spectra of several corneas at different levels of swelling, which may provide an alternative way to compare Raman signal to mechanical properties.
- Crosslinking of corneal collagen is of some interest as well. We will need to investigate if the Raman spectrometer is sensitive to changes in the of corneal collagen.
- In the end Professor Buckley is interested in finding a way to diagnose Keratoconus in human cornea before the onset of corneal thinning. Our goal is to develop a way of analyzing the mechanical properties of the cornea via Raman spectroscopy.
- Future: In vivo Raman imaging of cornea is also of some interest, however we are not sure if this can be/has been done before. Air pump test – correlation between pressures and mechanical properties?
Journal club paper for 2/5/16
Hi guys,
Here is the link to the Judy Mourant paper that we’ll be discussing at this week’s group meeting. Should be another good discussion, especially for us on the angular scattering project!
Paper talk Friday(01/22/2016)
Following is the paper we are going to talk about on Friday.
Differences in forward angular light scattering distributions between M1 and M2 macrophages
And if you have some notes or comments, you could comment in our blog, under this post!
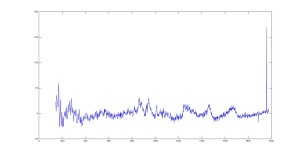
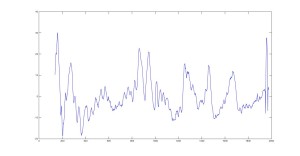
Raman Spectra of Cornea (Raw and Smoothed Data)
Notable Peaks
Amide III (protein): 1225-1275 cm-1
Amide I (protein): 1640-1675 cm-1
Phenylalanine (amino acid): 1003 cm-1
Tryptophan (amino acid): 760 cm-1 and 881 cm-1
Tyrosine (amino acid): 646 cm-1
CH2-CH3 bending band: 1275-1500 cm-1
Most peaks are accurately represented in our data, however some peaks seem to be shifted. Such as our Phenylalanine peak at 1010 cm-1 when it should be at 1003 cm-1. We believe this is an issue with the calibration from pixel to wavelength.
Questions regarding future evaluation of Ramen data of Cornea
1. How does the quality of our data set compared to what we expect to see out of Ramen signal from Cornea?
a. Set up two different data sets: one with raw data, and another one with smooth data (in matrix).
b. Set up a mean spectrum and calculate the trend of different peaks relative to the mean spectrum.
2. What biological information can we extract from the data set?
a. Hydration? Collagens? Mechanical Property? Chemical Property? Ratio of multiple peak strengths?
3. How do certain peaks change as we image different spatial positions or depth positions of sample?
a. Using the depth scanning / spatial actuator to quantify the peak strength.
4. Find publications regarding the area of interest in mechanical property of cornea.