Carbon is often treated as a “dirty” word in environmental circles. Carbon footprint, carbon emissions, carbon dioxide and carbon monoxide are all commonly discussed in environmental conversations. It’s true that these compounds should be avoided – carbon monoxide is poisonous after all. But carbon is much more than these terms imply. Carbon is the 15th most abundant element in the Earth’s crust, and the fourth most abundant element in the universe. In our bodies, carbon is the second most abundant element by mass (after oxygen). It is the chemical basis of all known life. The carbon system is fundamental to our health and livelihoods.
There is carbon in the earth’s atmosphere, all water bodies, and the biosphere. Our main sources of energy – hydrocarbons – are direct carbon compounds. One of the most important variations of carbon is carbon dioxide. Carbon dioxide at a moderate level is what causes the greenhouse effect and fuels photosynthesis. Indeed, carbon dioxide’s main purpose is to sustain plant life and keep us warm against the coldness of space. It is when carbon dioxide use is unfettered and abusived that problems arise.
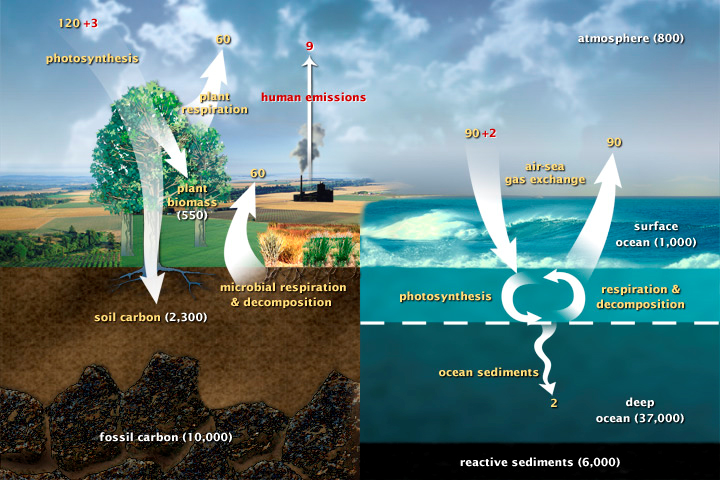
Here is a diagram of the carbon cycle. As you can see, carbon rotates through all aspects of the environment. The oceans are our main carbon sinks (where a particular element is absorbed and stored). The other major sink is terrestrial plants. Both these sinks do not absorb all the excessive amounts of carbon dioxide we inject into the atmosphere, causing the runaway greenhouse effect that is one of the most important issues of our lifetime.
So remember that carbon and its compounds are not bad – they protect and sustain you. But the next time you take out another graphite pencil, consider what you can do to reduce your negative carbon use.
By Alanna Scheinerman, Class of 2013
Related articles